Picea
A. Dietrich 1824
Common names
Spruce, épicéa (French), épinette (Canadian French), peccio (Italian), fichte (German), gran (Swedish, Norwegian & Danish), jel (Russian), swierk (Polish), smarch (Bulgarian), smrche or omorike (Serbo-croat), yunshan (Chinese), momi (Japanese).
Taxonomic notes
The following taxa are treated here:
Picea is related most closely to Pinus, but differs markedly even from that. It is a very uniform genus, clearly monophyletic with no aberrant species, so generic segregation has never been suggested (Farjon 1990), although historically it was subsumed within Abies for 70 years between the description of that genus by Miller in 1854 and the segregation of Picea by Dietrich in 1824. There are 35 species treated here. Classification within the genus was long problematic, and no satisfactory phylogeny was ever worked out despite numerous attempts using, mainly, morphological characters (Wright 1955, Bobrov 1970,
Liu 1982, Aldén 1987,
Page and Hollands 1987,
Rushforth 1987,
Schmidt 1989,
Farjon 1990, Frankis 1992,
Sigurgeirsson and Szmidt 1993). Molecular methods have been somewhat more useful; the phylogenetic tree at right summarizes the following analysis.
To date (2020) there have been three molecular studies, each sampling nearly all species in the genus, each using a very different approach:
- Ran et al. (2006) analyzed both paternal and maternal molecular markers (chloroplast and mitochondrial DNA), with multiple samples for a variety of east Asian taxa.
- Lockwood et al. (2013) used a broad suite of molecular markers (plastid, mitochondrial, and nuclear sequences), and had multiple samples for P. abies and two east Asian species. They also estimated a fossil-calibrated molecular clock.
- Shao et al. (2019) used a comparative transcriptomic analysis, and also derived a molecular clock.
The three studies were in broad agreement on several points, but diverged enough that it's difficult at this time to put confidence in a phylogenetic tree for all the species of Picea. In short, work to date has probably raised more questions than it has answered, but the three studies agree on the following points:
- P. breweriana appears in an outgroup, the earliest-divergent of all extant species of Picea, likely the sole survivor of an ancient Picea lineage that originated in North America and then dispersed to Asia, with all other North American species the product of one or more later dispersal events from Asia, back into North America.
- Within the other North American taxa, all agree on a sister relationship between P. engelmannii and P. glauca, and between P. mariana and P. rubens, and there is a general tendency to place these four species with other North American taxa (other than P. breweriana), but only Lockwood et al. (2013) place all North American taxa (other than P. breweriana) in a single clade.
- P. abies is sister to a group of (mostly northern) Asian species that have quadrangular needles. This group certainly includes P. asperata, P. crassifolia, P. koraiensis, P. meyeri, and P. retroflexa, but distinctions within that group are unclear. The three studies differ in the question of which Asian quadrangular-needled species are allied with that group, and how close the alliances are. In particular, there is very little agreement on placement of the Japanese species.
- All three studies identify an "other" group of east Asian spruces, i.e. the species that are neither quadrangular-leaved nor Japanese, which includes P. brachytyla, P. farreri, P. likiangensis, P. morrisonicola, P. neoveitchii, P. purpurea, P. schrenkiana, P. smithiana, P. spinulosa, and P. wilsonii. However, there is very little agreement on the relationships within this group; the studies don't even agree on the monophyly of infraspecific taxa of P. brachytyla and P. likiangensis.
After considering the above, here is what we know regarding the phylogenetic status of the remaining 14 species:
- The Japanese species (P. alcoquiana, P. glehnii, P. jezoensis, P. koyamae, P. maximowiczii, and P. torano) are all in Clade I of Lockwood et al. (2013), and all but P. torano and P. maximowiczii are in Clade III of Ran et al. (2006). They are widely scattered in the analysis of Shao et al. (2019). The morphological classification of Schmidt (1989) placed P. alcoquiana, P. koyamae, P. maximowiczii and P. torano into Series Picea, which otherwise includes the quadrangular-leaved species of east Asia, along with P. abies and P. obovata, and this result accords with what we know about the biogeography, history, and hybridization potential of this group of species. For two other Japanese species, P. glehnii and P. jezoensis, phylogenetic position is essentially unknown.
- P. obovata, as noted above, is generally thought to be closely related to P. abies, with a very extensive zone of introgressive hybridization where their ranges overlap west of the Ural Mountains; the hybrid is P. × fennica. This concept is supported by all three studies, but they differ in deciding which group these species are related to.
- P. aurantiaca is generally treated as a variety of P. asperata,and none of the molecular studies cited here have included a specimen of it.
- The Mexican species, P. chihuahuana, P. martinezii, and P. engelmannii subsp. mexicana, are supposedly closely related on biogeographical grounds. Ran et al. (2006) and Shao et al. (2019) did not evaluate two of these species, and had low confidence regarding the placement of P. chihuahuana. Lockwood et al. (2013) placed these three species with all the other North American species (other than P. breweriana) in their Clade II, a plausible result.
- P. pungens is treated by Ran et al. (2006) as sister to all the other species in their Clade III, not a very plausible result. Lockwood et al. (2013) place it within a clade of North American species, sister to P. engelmannii subsp. mexicana, an interesting result that is both biogeographically and morphologically plausible, though it seems not to have been proposed earlier. Shao et al. (2019) place it sister to P. chihuahuana, but with low similarity, in fact the lowest shown by any two sister taxa in their analysis.
- Ran et al. (2006) treat P. sitchensis as similar to P. breweriana; it is sister to a clade containing every other species in Picea, except P. breweriana. Lockwood et al. (2013) place it sister to the P. engelmannii-P. glauca clade, within a clade of North American species. Shao et al. (2019) place it sister to P. jezoensis, a result that makes very little sense.
- The last two species, P. omorika and P. orientalis, have similar biogeography, being located near each other in southeastern Europe and southwestern Asia, respectively. Ran et al. (2006) place them into different clades, Lockwood et al. (2013) place them in the same clade but sister to two Japanese species, and Shao et al. (2019) place them into different clades. For these two species, phylogenetic position is essentially unknown.
The studies by Lockwood et al. (2013) and Shao et al. (2019) both offered molecular clock analyses, which agreed in finding that all extant taxa have arisen since mid-Oligocene time, although there is a Jurassic age for the differentiation of Picea from Pinus. Also, the separation of P. breweri from the remainder of the genus likely occurred in the late Oligocene. Otherwise, radiation of the extant species mostly occurred over the whole of Miocene and Pliocene time, with a Pleistocene origin likely for, at most, only a few taxa.
Description
Evergreen trees; crown broadly conic to spirelike, 20-60 (-90) m tall; leading shoot erect. Bark gray to reddish brown, thin and scaly (with thin plates), sometimes with resin blisters, becoming relatively thick and furrowed with age. Branches whorled with strong nodal pseudowhorls and additional scattered weaker internodal branches; short (spur) shoots absent; twigs roughened by persistent leaf bases (pulvini). Buds ovoid, apex rounded to acute, sometimes resinous. Leaves borne singly, spreading radially from twigs, usually somewhat forward-pointing and often upswept, persisting to 10 years, mostly 4-angled and square in cross section (to triangular or ± flattened), mostly rigid, sessile on peglike base (called a sterigma); base decurrent, persistent after leaves shed, sheath absent; apex usually sharp-pointed, sometimes bluntly acute; resin canals 1-2. Cones borne on year-old twigs. Pollen cones single or grouped, axillary, oblong, yellow to purple; pollen shed in spring. Seed cones green to purple, maturing pale to dark brown in autumn, 4-8 months from pollination, usually shed at maturity, borne mostly on upper branches, pendent, ovoid to cylindric, sessile or terminal on leafy branchlets and thus appearing ± stalked; scales persistent, elliptic to fan-shaped, thin, lacking apophysis and umbo; bracts included. Seeds winged; cotyledons 5-10 (- l5). x =12 (Taylor 1993, M.P. Frankis e-mail 4-Jan-2002).
Distribution and Ecology
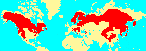
Restricted to subtropical high altitude , temperate, and boreal regions of the northern hemisphere. Confined to mountains in the south, its principal realm is the boreal forest, where it provides the dominant species across vast tracts of Scandinavia, Russia, Alaska, and Canada. It has its high est species diversity in the mountains of south and west China and Japan. The southernmost extension of the genus (P. morrisonicola) is on Taiwan, just south of the Tropic of Cancer at 23°N; in the New World Picea extends (via P. chihuahuana and P. martinezii) almost as far, but does not quite reach the Tropic (Taylor et al. 1994).
Remarkable Specimens
The largest trees in the genus are found among Picea sitchensis.
Maximum attainable ages are known for relatively few species. Working by analogy from other members of the Pinaceae, the greatest ages are normally found on sites that provide extreme physiological stress due to drought, cold, or darkness (i.e. deep shade). Picea avoids arid climates, but it encounters cold stress and some drought stress at alpine timberline sites. An age of 852 years recorded for P. engelmannii on such a site is evidently the current record for the genus.
Ethnobotany
The genus is of major economic importance for timber, the most important species being P. sitchensis and P. abies. Several species are commonly used for Christmas trees, most often P. abies but also P. omorika and P. mariana (surely one of the homeliest conifers).
Observations
Remarks
Picea is derived from the Roman pix, pitch (Weber 1987); or from picis, the name of a pitchy pine (Taylor 1993).
Citations
Aldén, B. 1987. Taxonomy and geography of the genus Picea. International Dendrological Society Yearbook 1986: 85-96.
Dietrich, A. 1824. Flora der gegend um Berlin; oder, Aufzählung und beschreibung der in der mittelmark wild wachsenden und angebauten pflanzen. Mit einer vorrede begleitet von H.F. Link. Theil i. Phanerogamen. Berlin. Available at HathiTrust, accessed 2020.11.26.
Farjon, Aljos. 1990. Pinaceae: drawings and descriptions of the genera Abies, Cedrus, Pseudolarix, Keteleeria, Nothotsuga, Tsuga, Cathaya, Pseudotsuga, Larix and Picea. Königstein: Koeltz Scientific Books.
LePage, B.A. 2001. New species of Picea A. Dietrich (Pinaceae) from the middle Eocene of Axel Heiberg Island, Arctic Canada. Biol. J. Linn. Soc. 135:137-167.
Lockwood, Jared D., Jelena M. Aleksic, Jiabin Zou, Jing Wang, Jianquan Liu, and Susanne S. Renner. 2013. A new phylogeny for the genus Picea from plastid, mitochondrial, and nuclear sequences. Molecular Phylogenetics and Evolution 69:717-727.
Ran, J.-H., Wei, X.-X. and Wang, X.-Q. 2006. Molecular phylogeny and biogeography of Picea (Pinaceae): Implications for phylogeographical studies using cytoplasmic haplotypes. Molecular Phylogenetics and Evolution 41(2):405-419.
Shao, Cheng-Cheng, Ting-Ting Shen, Wei-Tao Jin, Han-Jie Mao, Jin-Hua Ran, and Xiao-Quan Wang. 2019. Phylotranscriptomics resolves interspecific relationships and indicates multiple historical out-of-North America dispersals through the Bering Land Bridge for the genus Picea (Pinaceae). Molecular phylogenetics and evolution 141: 106610.
Stöber, Kurt. 1999. Images from Thomé, Flora von Deutschland etc. http://www.mpiz-koeln.mpg.de/~stueber/thome/index.html, accessed 2003.05.01, now defunct.
Wilson, L.R., and R.M. Webster. 1946. Plant microfossils from a Fort Union Coal of Montana. American Journal of Botany 33:271-278.
See also
Roche, L. 1969. A genecological study of the genus Picea and seedlings grown in a nursery. New Phytologist 68: 505-554.
Taylor, R.J. and T.F. Patterson. 1980. Biosystematics of Mexican spruce species and populations. Taxon 29: 421-469.