Tsuga mertensiana
(Bongard) Carrière 1867
Common names
Mountain hemlock, black hemlock, hemlock spruce (Peattie 1950), pruche de Patton (Taylor 1993).
Taxonomic notes
The sole member of Tsuga subgenus Hesperopeuce (Lemmon) Ueno, told from subgenus Tsuga by the long cylindric cones with pubescent scales, and the less flattened leaves with stomata on both sides.
Synonymy (Taylor 1993):
- Pinus mertensiana Bongard 1832;
- Hesperopeuce mertensiana (Bongard) Rydberg;
- A. pattoniana Jeffrey ex Balfour;
- Tsuga pattoniana (Balfour) Sénéclause;
- Pinus pattoniana (Balfour) Parlatore; and
- Hesperopeuce pattoniana (Balfour) Lemmon.
Two subspecies, the type and T. mertensiana subsp. grandicona Farjon (Farjon 1988, Farjon 1990). One poorly-differentiated variety, T. mertensiana subsp. mertensiana var. jeffreyi (Henry) Schneider [syn. Tsuga pattoniana var. jeffreyi Henry]. The variety has also been considered a hybrid with T. heterophylla, as T. × jeffreyi (Henry) Henry, but the cones show no sign of influence of that species, and hybridisation has never been verified experimentally (Taylor 1972, Farjon 1990, Taylor 1993).
M. Van Campo-Duplan and H. Gaussen in 1948 postulated that this taxon originated by hybridization between Picea and Tsuga. This hypothesis has recently been disproved by DNA studies; X-R Wang (in press) (M.P. Frankis e-mail 1999.02.06).
Description
Trees to 40 m tall and 150 cm dbh; crown conic. Bark charcoal gray to reddish brown, scaly and deeply fissured. Twigs yellow-brown, densely pubescent. Buds oblong, 3-4 mm. Needles 10-25(30) mm, mostly spreading in all directions from twigs, curved toward twig apex, thickened centrally along midline, somewhat rounded or 4-angled in cross section, both surfaces glaucous, with ± inconspicuous stomatal bands; margins entire. Seed cones purple ripening mid to dark grey-brown, oblong-cylindric, 3-6 × 1.5-2.5 cm (open); scales pubescent, broadly fan-shaped, 8-l1 × 8-11 mm, apex rounded to pointed. 2n=24 (Taylor 1993, M.P. Frankis e-mail 1999.02.06).
Var. jeffreyi differs in sparser leaves, greener with fewer stomata above, possibly an adaptation to lower altitude habitats, but it is as yet poorly researched. In the past, the greener leaves have been interpreted as being due to hybridisation with T. heterophylla, but the cones and growth habit are indistinguishable from typical
T. mertensiana (M.P. Frankis e-mail 1999.02.06).
Distribution and Ecology
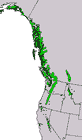
Canada: British Columbia; USA: Alaska, Washington, Idaho, Montana, Oregon, California and Nevada at 0-3050 m. The elevational range of the species is quite variable with latitude, ranging from 0-1070 m in Alaska, to 1600-2300 m in southern Oregon (where subsp. mertensiana grades into subsp. grandicona), to 2750-3050 m in the southern Sierra Nevada. See also Thompson et al. (1999). Hardy to Zone 4 (cold hardiness limit between -34.3°C and -28.9°C) (Bannister and Neuner 2001).
Throughout the range of this species, mean annual temperature is approximately constant at 3-4°C and precipitation, though highly variable (970 to 3020 mm), does not show a latitudinal gradient. However, the portion of precipitation falling as snow decreases greatly with latitude, from 88 percent in California to 14 percent in Alaska, reflecting a translation from Mediterreanean to subarctic maritime climates across 25 degrees of latitude. Its habitat is relatively coastal; it does not occur in the rainshadow of the Coast-Cascade-Sierra Nevada mountain chain except locally in the Rocky Mountains where coastal airmasses can penetrate and produce high winter snowfall. It has been suggested that the species affinity for areas with persistent winter snowpacks is because it cannot tolerate frozen soils. Throughout most of its range, T. mertensiana defines the upper portion of the subalpine forest and is conventionally divided into two elevational zones: the forest subzone of continuous closed-canopy forest, and the parkland subzone of tree clumps separated by fellfields, wet meadows or low subalpine shrubs. Within the parkland subzone, the discontinuous forest canopy typically reflects reduced seedling establishment success associated with a deep and persistent winter snowpack. At the highest elevations, T. mertensiana grows as a timberline tree in krummholz form (Arno and Gyer 1973, Burns & Honkala 1990, Taylor 1993).
Var. jeffreyi has been reported from a few scattered sites within the range of the type (Farjon 1990).
The two lower photos at right illustrate some of the principal features of T. mertensiana tree clumps in the subalpine parkland. These clumps often contain one or two exceptionally large and old trees, along with several younger trees. The younger trees commonly represent several different age classes. The oldest tree may not date the origin of the tree clump; often the decayed remains of still older trees can be found within the clump. The origin and development of these clumps has been studied by various authors; a literature review appears in my dissertation (Earle 1993). Arno and Hammerly (1984) also provide an informative discussion of the subject. Typically these clumps are located on topographic high points where winter snows melt out marginally earlier than on adjacent microsites. Establishment of trees on these sites is commonly correlated with episodes of relatively warm, dry climate that last several years; in the Cascades, for instance, such episodes were recorded in the 1930s (Franklin et al. 1971) and again in the latter 1990s (pers. obs.). Seedlings established in the 1990s are still very small and it will be interesting to see how they fare under global warming. Once a clump contains at least one tree tall enough to project above the winter snowpack, blackbody radiation emitted from the tree causes the snow to melt sooner and faster near the tree than in the open meadow. In this way the tree alters its environment to reduce snow accumulation and produce conditions conducive to both the growth of existing trees and the establishment of new trees, both from seed and via asexual means (layering) (Earle 1993). Over time, the tree clump tends to grow radially; such clumps have been called "timber atolls" (Griggs 1938). The formation of such clumps is not confined to T. mertensiana. Many timberline species have been observed to form clumps in areas where a deep and persistent snowpack retards tree establishment, among them, Abies amabilis, Abies lasiocarpa, Picea engelmannii, Pinus albicaulis, and Callitropsis nootkatensis. It is also not unusual to find more than one species of conifer in a clump. T. mertensiana typically initiates clumps within its range, but such clumps often also support Abies amabilis or Callitropsis nootkatensis. On drier sites, such as in the Rocky Mountains, clumps are commonly initiated by Pinus albicaulis, and a "skirt" of young Abies lasiocarpa then grows up around it. After a period of centuries, the Pinus may die due to shading by the now-tall Abies, and the Abies may in its time give way to more shade tolerant Picea engelmannii. Given enough time and a favorable climate, clumps may eventually coalesce to form a continuous forest, but an episode of severe cold/wet weather or a catastrophic fire can reset the clock, returning the site to an open meadow.
Remarkable Specimens
In 1996, the official 'Big Tree' was a specimen of subsp. grandicona 34 m tall with a dbh of 224 cm and crown spread of 13 m, in Alpine County, CA (American Forests 1996). As of 2021, the official big tree is considerably smaller, 197 cm dbh and 27.7 m tall with a crown spread of 15 m. In 1998, the largest known specimen of subsp. mertensiana was 59.1 m tall, with a dbh of 127 cm, in Olympic National Park, WA (Robert Van Pelt (who measured this tree) e-mail 1998.03.18). I have seen, but not precisely measured, trees of subsp. mertensiana at least this big; while hiking the Pacific Crest Trail in Washington in 2021, I saw a tree 178 cm dbh and approximately 30 m tall north of Pear Lake, and another tree approximately 200 cm dbh and 20 m tall at Wards Pass (photo at right).
Muir (1894) described a tree 19 feet 7 inches in girth (191 cm dbh) near Lake Hollow in the Sierra Nevada; this is one of the earliest big tree measurements recorded.
Parish and Antos (2004) analyzed age structures for mountain hemlocks in northern Vancouver Island, British Columbia and found one specimen 1059 years old (measured and crossdated); this was from a stump in a clearcut. The same site also produced some of the oldest known Abies amabilis and Callitropsis nootkatensis (also stumps in a clearcut). Another very old living specimen, 722 years, was documented in a tree-ring chronology covering the period 1188-1990 (crossdated after 1370), collected in the Three Sisters Wilderness, Oregon, by Emily Heyerdahl (doi.org/10.25921/hthn-eb42).
Ethnobotany
The wood of Tsuga mertensiana is somewhat inferior to that of T. heterophylla as timber and as pulp, a fact that has little retarded extensive logging of subalpine forests. It is adaptable to a wide variety of climatic conditions and is widely used as an ornamental (Burns & Honkala 1990) (USDA hardiness zone 5). In this capacity it differs from many alpine conifers in its tolerance of relatively warm, damp environments, and in such settings remains healthy while growing slowly; ideal characteristics for an ornamental conifer.
This species has been useful in dendrochronology. As of February 1999, there were about 25 published studies dating as far back as 1923. The great majority of these studies have examined climate or some factor closely related to climate, such as timberline fluctuation or glacier expansion. The utility of the species for climate studies is due to its occurrence at the alpine timberline and its strong interaction with snowpack accumulation. For instance, it is by now generally accepted that widespread invasion of subalpine meadows in the Pacific Northwest happened during the 1930s and 1940s in response to a prolonged episode of reduced winter snowpacks (Franklin 1988). Graumlich and Brubaker (1986) looked at the relationship between climate and ring width for some stands in the Cascade Range of WA, and I did some (unpublished) exploratory studies focusing on population age structures and competitive interactions in subalpine parklands of the Cascade Range of BC, WA and OR.
Observations
Because this species grows to the alpine timberline, its most picturesque qualities are displayed in high mountain areas. Notably good sites are the parklands, where the landscape is covered by a mosaic of meadow and tree clumps. Examples include the high country of Garibaldi Provincial Park in BC, the Seven Lakes Basin of Olympic National Park in WA, and Jefferson Park in the Mount Jefferson Wilderness of OR. Subsp. grandicona can also be found growing nearly everywhere in the high alpine lake country of the Sierra Nevada, including areas in Yosemite, Kings Canyon and Sequoia National Parks.
Remarks
The epithet honors Karl Heinrich Mertens (1796-1832), a German botanist remembered primarily for collections he made as botanist on the Lutke around-the-world expedition, which included this species. Though Mertens died shortly after his return to Europe, his plant collections were cataloged by A. H. von Bongard, who named this species in his honor. For more about Mertens, see Mirov (1954).
Citations
American Forests 1996. The 1996-1997 National Register of Big Trees. Washington, DC: American Forests.
Arno, Stephen F. and Jane Gyer. 1973. Discovering Sierra trees. Yosemite Natural History Association. 89pp.
Arno, Stephen F. and Ramona Hammerly. 1984. Timberline: mountain and arctic forest frontiers. Seattle: The Mountaineers.
Farjon, Aljos. 1988. Taxonomic notes on Pinaceae 1. Proc. Konin. Ned. Akad. Wetensch. ser. C Bot., 91: 31-42.
Farjon, Aljos. 1990. Pinaceae: drawings and descriptions of the genera Abies, Cedrus, Pseudolarix, Keteleeria, Nothotsuga, Tsuga, Cathaya, Pseudotsuga, Larix and Picea. Königstein: Koeltz Scientific Books.
Franklin, J. F., W. H. Moir, G. Douglas, and C. Wiberg. 1971. Invasion of subalpine meadows by trees in the Cascade Range. Arctic and Alpine Research 3(3): 215-224.
Franklin, Jerry F. 1988. Pacific Northwest forests. P. 103-130 in M.G. Barbour and W.D. Billings, eds., North American terrestrial vegetation. Cambridge: Cambridge University Press.
Graumlich, Lisa J. and Linda B. Brubaker. 1986. Reconstruction of annual temperature (1590-1979) for Longmire, Washington, derived from tree rings. Quaternary Research 25:223-234.
Griggs, R. F. 1938. Timberlines in the northern Rocky Mountains. Ecology 19:548-564.
Mirov, N. T. 1954. Lodgepole pine discovered and misnamed. Madroño 12:156-157. Available: Biodiversity Heritage Library, accessed 2023.07.17.
Muir, John. 1894. The Mountains of California. Available: http://yosemite.ca.us/john_muir_exhibit/writings/the_mountains_of_california/index.html, accessed 2019.02.04.
Parish, Roberta, and Joseph A. Antos. 2004. Structure and dynamics of an ancient montane forest in coastal British Columbia. Oecologia 141(4):562-576.
Taylor, R. J. 1972. The relationship and origin of Tsuga heterophylla and Tsuga mertensiana based on phytochemical and morphological interpretations. American Journal of Botany 59: 149-157.
See also
Brooke, R. C., E. B. Peterson, and V. J. Krajina. 1970. The subalpine mountain hemlock zone. Ecology of western North America 2:151-307.
Elwes and Henry 1906-1913 at the Biodiversity Heritage Library (as T. pattoniana) (Photo). This series of volumes, privately printed, provides some of the most engaging descriptions of conifers ever published. Although they only treat species cultivated in the U.K. and Ireland, and the taxonomy is a bit dated, still these accounts are thorough, treating such topics as species description, range, varieties, exceptionally old or tall specimens, remarkable trees, and cultivation. Despite being over a century old, they are generally accurate, and are illustrated with some remarkable photographs and lithographs.
Lanner 1983.
MacKinnon et al. 1992.
Sargent (1898) provides an exceptionally detailed description of this species, with an excellent illustration.